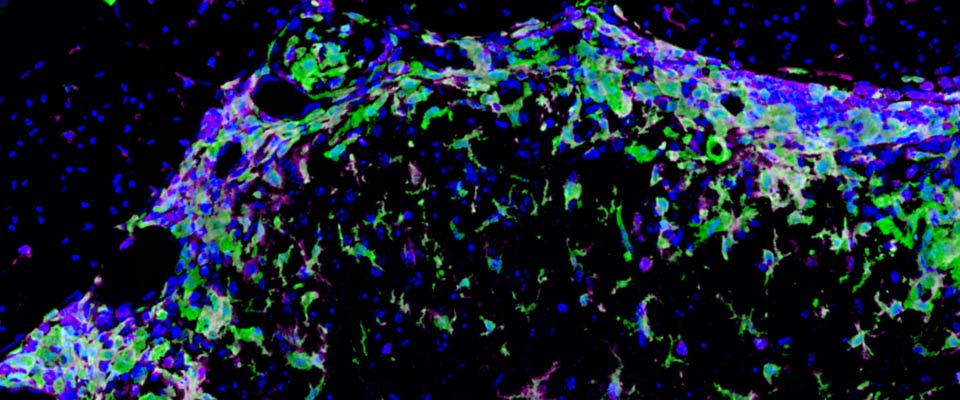
In published studies we have examined the role of myeloid cells during multiple sclerosis (MS) and the animal model, experimental autoimmune encephalomyelitis (EAE). We have found that CNS-infiltrating myeloid cells are heterogeneous, and include monocytes, macrophages, classical dendritic cells, and myeloid dendritic cells. Macrophages and myeloid dendritic cells transition from a pro-inflammatory iNOS+ phenotype during disease exacerbation, to an immune-quiescent Arginase-1+ phenotype during remission. We are currently investigating the role of myeloid cell subsets in phagocytosing myelin, releasing toxic factors and activating pathogenic autoreactive T cells at peak disease, as well as in inducing regulatory T cells, promoting remyelination, and stimulating axon regeneration during recovery.
Ongoing Projects:
- Interrogation of the growth factors, cytokines, chemokines and adhesion molecules that drive the differentiation of pro-regenerative neutrophils in vivo and orchestrate their migration to sites of CNS injury
- Further characterization of the mechanism of action of pro-regenerative neutrophils and translation of the knowledge gained towards the development of novel reparative therapies for chronic CNS diseases
- Generation of pro-regenerative neutrophils from bone marrow precursors in vitro (via culture with cytokine cocktails) for re-infusion to individuals with CNS injury as an autologous cellular therapy
- Assessment of the therapeutic potential of pro-regenerative neutrophils in spinal cord and brain trauma models
- Investigation of human pro-regenerative neutrophils
Related Publication:
- Baldwin KT, Carbajal KS, Giger RJ, Segal BM. Neuroinflammation triggered by a β-glucan/ dectin-1 signaling enables CNS regeneration. Proc Nat Acad Sci 2015;112(8): 2581-6. PMID: 25675510
Related Funding:
- National Institutes of Health (R01EY029159)
A novel inflammatory cell with neuroprotective and neuroregenerative properties - National Institutes of Health (R01EY028350)
Immune mediated regeneration of retinal ganglion cell axons following optic nerve trauma
