
The Ohio State University Wexner Medical Center expands treatment options for patients with COPD
 Researchers at The Ohio State University are collaborating with industry partners, who may receive up to $7.75 million in funding to develop and implement an innovative algorithm for early detection of sepsis in all hospital wards, including emergency departments (EDs).
Researchers at The Ohio State University are collaborating with industry partners, who may receive up to $7.75 million in funding to develop and implement an innovative algorithm for early detection of sepsis in all hospital wards, including emergency departments (EDs).
Their efforts – which build on five years of research to validate a novel biomarker that can quickly identify septic patients – are poised to transform sepsis diagnosis, potentially saving millions of lives worldwide.
In the United States alone, around 1.7 million adults develop sepsis every year. This results in nearly 270,000 deaths annually.
“In approximately 80% of sepsis cases, sepsis is present on admission to the ED,” says Ohio State pulmonologist and critical care medicine specialist Elliott Crouser, MD. “Unfortunately, current clinical parameters used to diagnose sepsis, including symptom evaluation and blood tests, often lead to delayed recognition and treatment due to inability to rapidly differentiate sepsis from other acute illnesses. And the timing of sepsis detection and treatment is important because for every hour that passes without treatment, there is a 5 to 10% increase in mortality.”
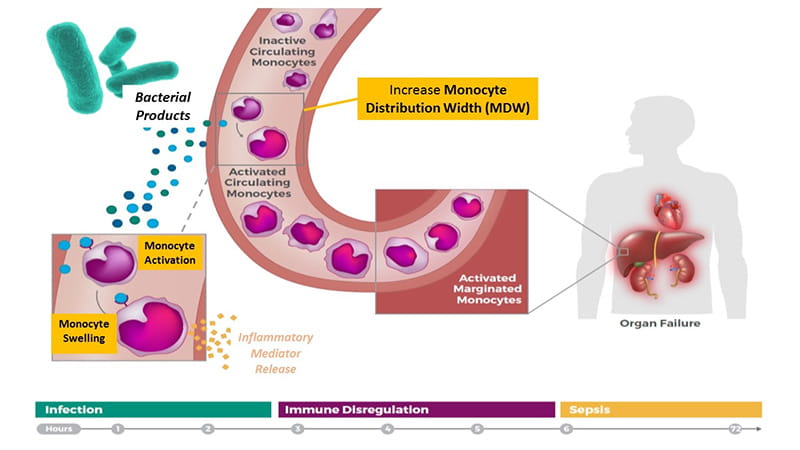
In 2014, Dr. Crouser led an industry-sponsored feasibility study at Ohio State to determine if volume increases in circulating immune cells correlate with sepsis manifestation.
“One of the first signs that a localized infection is transitioning to sepsis is immune cell activation in the circulation,” Dr. Crouser says. “Because monocytes are among the first responders, we tested for increases in monocyte volume and confirmed whether volume changes corresponding with activation were predictive of sepsis.”
Their results were published in CHEST in September 2017. They showed that a biomarker called monocyte distribution width (MDW) – which measures a change in the size distribution of circulating monocytes – provides significant added value to white blood cell (WBC) count, a common lab test used to initially screen for sepsis, in terms of improved detection of sepsis in the ED population.
Following the feasibility study, Dr. Crouser led two multicenter trials for Beckman Coulter to gather more data about the diagnostic accuracy of MDW alone and in combination with WBC count. Together with researchers from the University of Pittsburgh and Hackensack University Medical Center, Dr. Crouser’s team enrolled 2,158 subjects in their pivotal study.
Those results, featured in Critical Care Medicine in August 2019, confirmed that an increase in MDW effectively identifies patients who are septic and patients with infections who are at increased risk for progression to sepsis.
“We also showed that the MDW parameter works synergistically with all the other clinical parameters available at the initial ED encounter,” Dr. Crouser says. “When the white blood cell count is abnormal along with MDW, there is a higher risk of sepsis. And if the patient also has fever and tachycardia, the MDW further refines diagnostic accuracy.”
In May 2019, the Food and Drug Administration cleared Beckman Coulter’s MDW biomarker’s use for early detection of sepsis in the ED setting. Beckman Coulter, Inc., will now move this novel laboratory test – named the Early Sepsis Indicator (ESId) – into the next phase of research.
In September of 2019, the Biomedical Advanced Research and Development Authority’s (BARDA) Division of Research Innovation and Ventures (DRIVe), part of the HHS Office of the Assistant Secretary for Preparedness and Response, obligated $1.25 million under a cost-sharing contract with Beckman Coulter. BARDA DRIVe could obligate an additional $6.5 million over four years under the cost-sharing contract to support advanced research and development, validation and regulatory approval of the novel algorithm-based diagnostic. Beckman Coulter will work with Dascena Inc. of Oakland, California, to develop the algorithm.
BARDA oversees an initiative that brings together academic and industry leaders to reduce the incidence, morbidity, mortality and cost of sepsis. The Ohio State University (Dr. Crouser) will work with Beckman Coulter to combine the Early Sepsis Indicator with a novel algorithm that has been shown to predict sepsis using data from patients’ electronic health records (EHRs).
“For the next phase of research, Beckman Coulter has partnered with Dascena, Inc., a company that develops algorithms that predict complex conditions in inpatient and emergency settings,” Dr. Crouser says. “They’ve developed a machine-learning algorithm that can detect sepsis using patient vital sign data.”
Dr. Crouser is collaborating with researchers from other US hospitals to further develop this effort. The aim is to determine whether MDW and WBC data can be integrated with Dascena’s EHR-based algorithm to achieve unprecedented sepsis prediction – potentially providing a warning hours before a patient becomes septic. The team is actively pursuing a Software as a Medical Device (SaMD) FDA regulatory approval path for the algorithm.
Photo credit: Beckman Coulter Inc.
Learn more about innovations in care and research from the Division of Pulmonary, Critical Care and Sleep Medicine.