Comprehensive Esophageal Health Center opens with more convenient, streamlined care
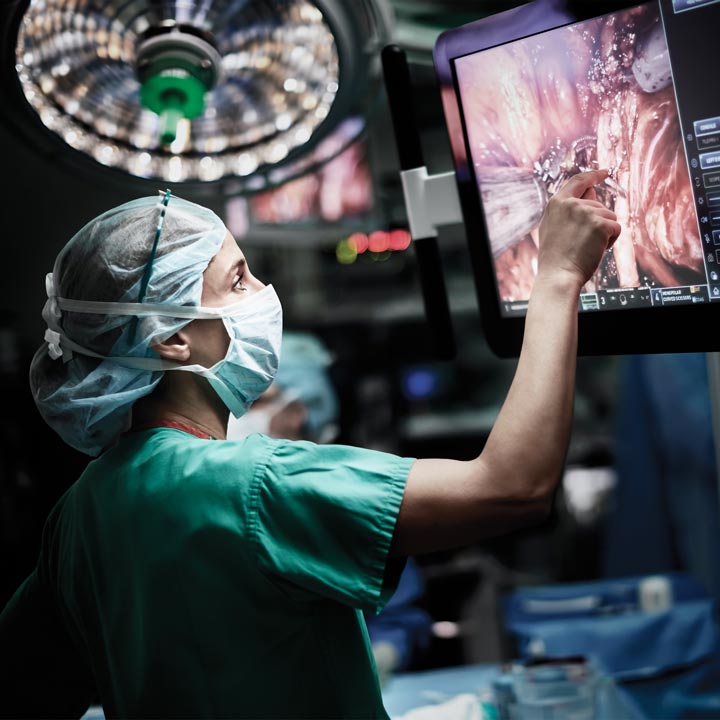 Anastomotic leak is one of the most serious risks after colorectal surgery. But now, researchers at The Ohio State University are working to improve those outcomes by conducting the first human-patient clinical trial with ActivSight, a new FDA-approved device that assesses blood flow (tissue perfusion) in surgery and provides enhanced surgical visualization.
Anastomotic leak is one of the most serious risks after colorectal surgery. But now, researchers at The Ohio State University are working to improve those outcomes by conducting the first human-patient clinical trial with ActivSight, a new FDA-approved device that assesses blood flow (tissue perfusion) in surgery and provides enhanced surgical visualization.
“The goal of the study is to better determine the safety and feasibility of ActivSight, and to begin understanding how such a device might impact colon anastomoses,” says Emily Huang, MD, MEd. Dr. Huang is an assistant professor of Surgery in the Ohio State College of Medicine and vice chair for Education at The Ohio State University Wexner Medical Center.
There are some situations in which it would help surgeons tremendously to have more information about blood supply. That supply brings nutrients and healing to the area where the colon was connected during surgery.
ActivSight, from Activ Surgical, attaches to a standard laparoscopic camera using laser speckle contrast imaging (LSCI) technology to detect how quickly particles are moving in various areas of the laparoscopic image. In LSCI, coherent light scatters back from tissue and speckles are produced, which creates an image. This allows surgeons to see where blood is flowing.
Being able to visualize and assess blood flow is important because some types of colon anastomosis, especially colorectal anastomoses, are at high risk for leakage. So having information that may help with decisions during surgery would prove extremely useful to colorectal surgeons.
“We can use the ActivSight device to check blood flow before and after making connections in the colon,” Dr. Huang says, “and to make sure the colon will heal well and not leak.” Although anastomotic leaks are fairly rare – occurring in about 5% of anastomosis surgeries – they can be dangerous, as they can result in colon contents leaking into the abdominal cavity and causing complications such as infections.
Traditionally, surgeons completing a colorectal resection would visually assess the connection in the colon. If it looked like it was bleeding or pink, they knew there was a major blood vessel going to the area.
Then another way to assess the colon was developed. An anesthesiologist would administer ICG dye into the bloodstream during surgery, and wherever the dye went, tissue was perfused. But the significant drawback was the risk of the patient negatively reacting to the dye. A typical adverse reaction would involve kidney function.
The ActivSight device that Ohio State researchers are studying and testing piggybacks onto a camera. Laser speckle is used to assess blood flow. This eliminates the need to inject dye and the risk that comes with it. Between the camera view and laser speckle, views can be overlaid to better assess perfusion.
Some organizations have already completed extensive animal studies on the ActivSight device. They found the technology is useful for many applications, including visualizing the bowel duct and ureters.
“Using this technology is promising because it can be overlapped with the clinical view in real time,” Dr. Huang says, “and the surgeon can alternate between the clinical view and using this technology.” In addition, using ActivSight is extremely cost-effective and safe. The study introduces minimal risk to patients, as they’re already undergoing surgery for anastomosis, so surgeons just add ActivSight visualization.
“This is amazing,” Dr. Huang says.
“Ohio State has a strong commitment to research that furthers clinical expertise,” Dr. Huang says, “and we have faculty and a system that’s highly supportive of doing studies like we are doing.”
The intention at the outset of the study was to enroll 40 patients over a year, and the research team is well on its way to accomplishing that. Because Ohio State faculty and staff complete a high volume of minimally invasive colorectal procedures, we have an ideal location to complete this type of research.
“We are looking to demonstrate that the device itself is safe and feasible for use in colorectal anastomoses,” Dr. Huang says, “and if so, it may also be useful for other applications, such as assessing tissue perfusion.” If the device proves feasible for checking perfusion, it may help to improve bowel anastomoses.
The research team is finding that ActivSight is beneficial as an adjunct in laparoscopic as well as robotic surgery, and it’s minimally invasive.