
Scientists gain large NIH grant to study cancer treatment links to heart disease
The Ohio State University Heart and Vascular Center’s expert physicians and staff can treat any cardiovascular condition, and our researchers are continually turning today's science into tomorrow's treatments. Read about the latest clinical and research innovations taking place at the Ohio State Heart and Vascular Center.
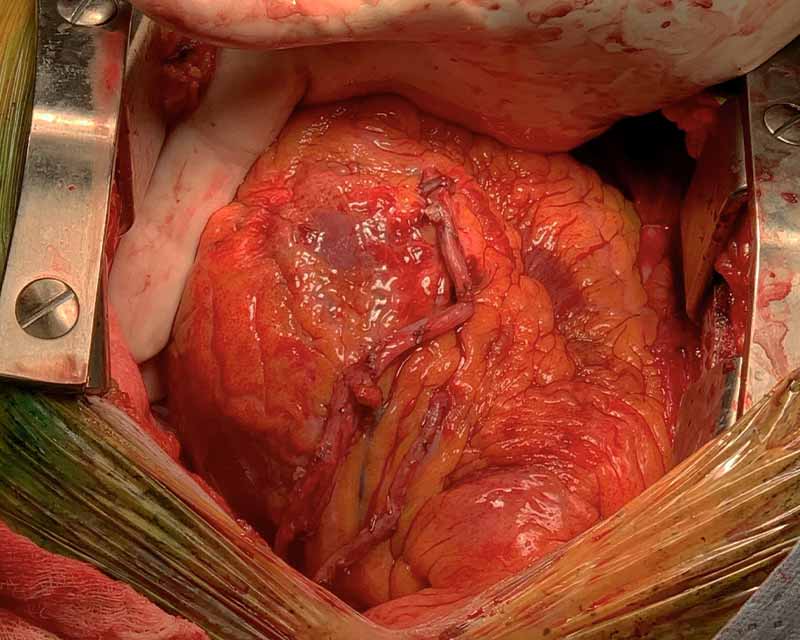
Achieving better long-term outcomes for patients with coronary artery disease
Ohio State first in nation to use new treatment for heart failure patients

A pivotal clinical trial to address the unmet need of HFrEF patients

Safer Patient Transport

New treatment for atrial fibrillation
First US Medical Center in Clinical Trial

Lead on new therapies for cardiovascular disease
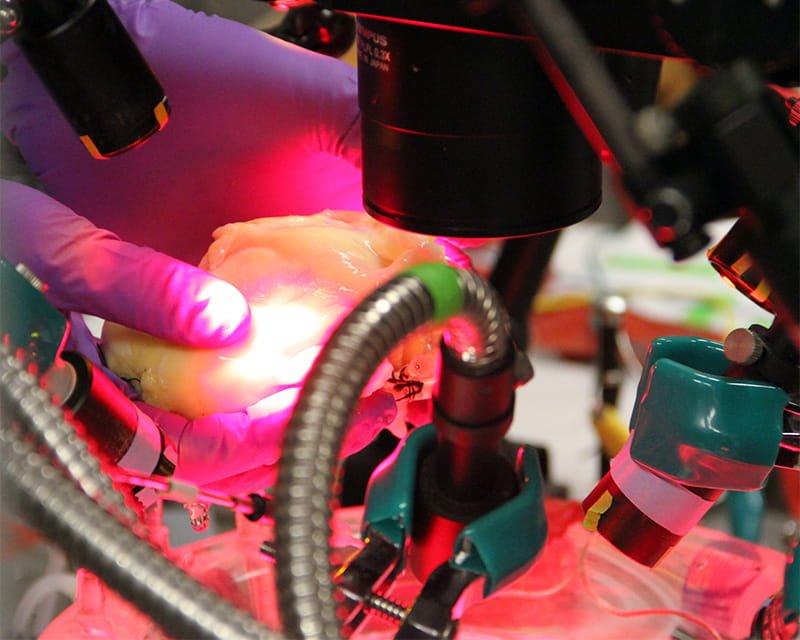
Advancing Research with 3D Imaging

Ohio State Launches Cardiogenic Shock Protocols
Testing Heart Perfusion

Review of current research calls for better studies
Ohio State Structural Heart Disease Program

Cardiac Palliative Care Program
Advancing Care for Aortic Disease

Davis Heart and Lung Research Institute
The Ohio State JB Project

Ohio State Inherited Arrhythmia Clinic

Ohio State Adult Congenital Heart Disease Program

Ohio State Heart Genetic Research

Leading the way