Ohio State provides lifelong care for inflammatory bowel disease
The Division of Gastroenterology, Hepatology and Nutrition, the Division of General and Gastrointestinal Surgery and the Division of Colon and Rectal Surgery are working together to provide the latest treatment options to patients suffering from digestive diseases. Our physicians and researchers continuously look for new ways to prevent chronic illness and cancer through early detection like routine screenings, and breakthrough research that might identify new markers for disease that weren’t previously known.
U.S. News & World Report ranks Ohio State 45th among the best hospitals in the nation for gastroenterology and GI surgery.
These stories highlight some of the most promising work in our field.

Ohio State provides lifelong care for inflammatory bowel disease

Ohio State expands colorectal cancer screening with free colonoscopy program
Ohio State advancing the field of endoscopy through unique care delivery, research and novel procedures

Nonsurgical endobariatrics offered as part of Ohio State’s Comprehensive Weight Management program

Ohio State offers world’s first colorectal transitional care clinic
New Colorectal Cancer Center aims to bolster patient care, become regional and national treatment destination

How Ohio State APPs elevate patient care
Sheryl Pfeil, MD, joins AGA Governing Board, continuing her educational mission

Michael Meara, MD, performs 1,000th robotic operation
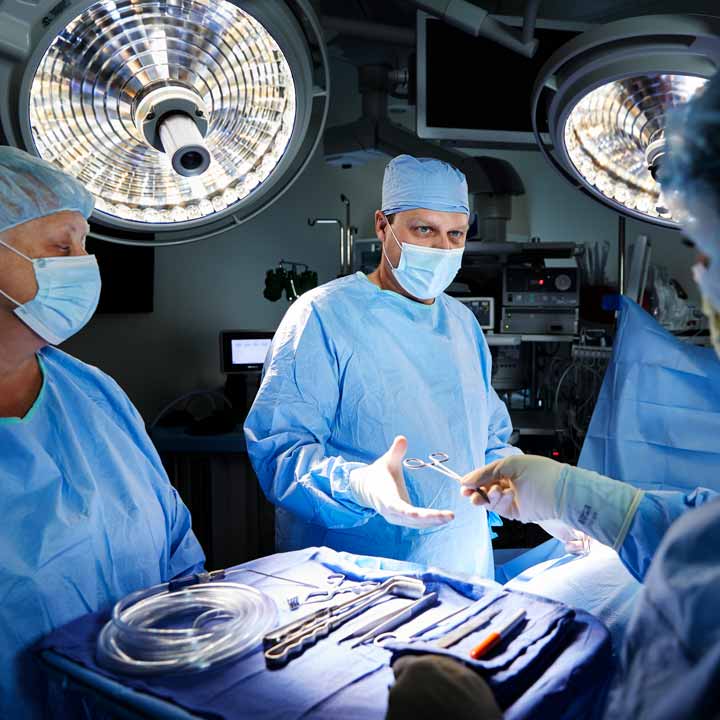
New outpatient surgery center combines advanced treatments with fast-track patient access to care

Hereditary Colorectal Cancer Syndrome Clinic advances collaborative prevention and screening

NIH awards Ohio State two multicenter grants to advance research of pancreatitis, diabetes and pancreatic cancer
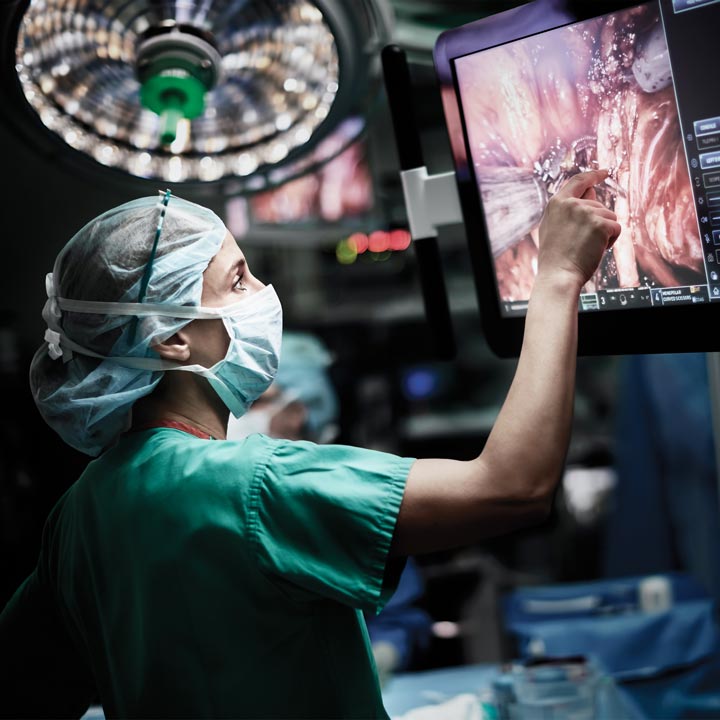
Enhanced surgical visualization device that assesses blood flow shows promise for improving colorectal surgery outcomes
.jpg?la=en&hash=C875325045FF6FF6C0AA43825A9FFA4F)
New gastroenterology director eyeing significant growth in patient care, education and research

Leading the charge toward improving geriatric patient outcomes

Internationally known physician developing neurogastroenterology and motility program
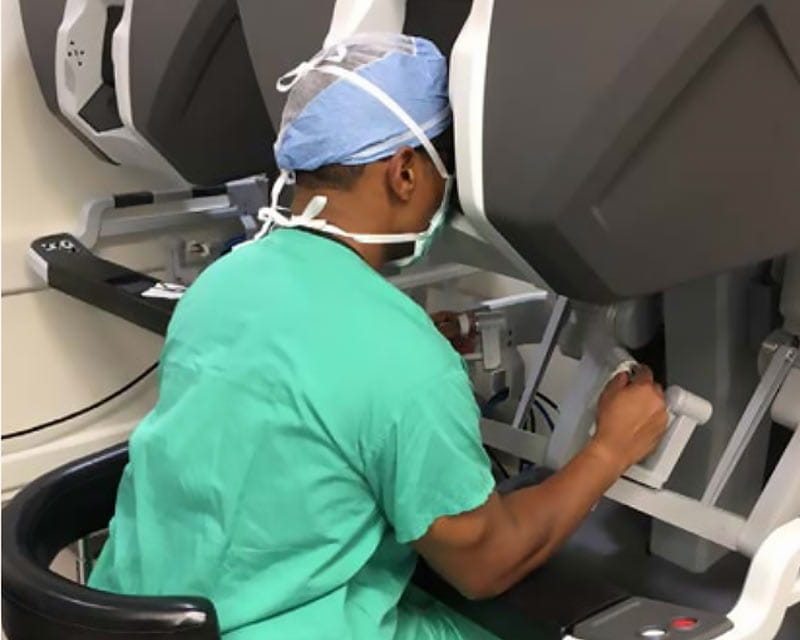
Applying robotic techniques to bariatric surgeries

Treating pelvic floor-related bowel conditions with convenient, streamlined patient care

Anal cancer screening program launching at Ohio State

Physical therapy for older hernia patients: NIH grant funds Ohio State research
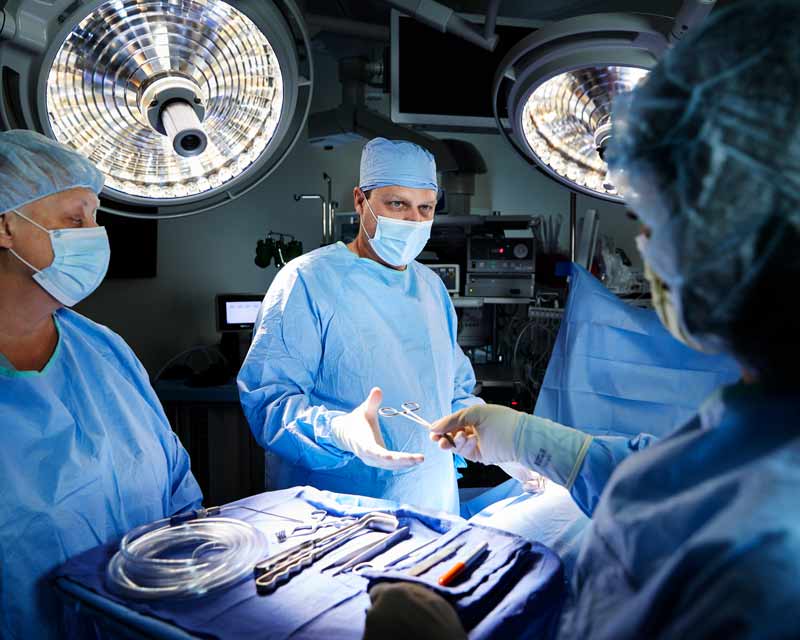
Comprehensive Esophageal Health Center opens with more convenient, streamlined care

An Ohio State physician describes an endoscopic ultrasound (EUS)-guided gastrojejunostomy
Advanced GI cancer patients find new hope at Ohio State

Unlocking the mysteries surrounding acute pancreatitis and diabetes

Ohio State basic scientist researches role of key proteins in pancreatic cancer
Ohio State liver transplant program nationally ranked in volume

Matthew Kalady, MD, named division director of Colon and Rectal Surgery
An Ohio State physician describes an endoscopic ultrasound (EUS)-guided choledochoduodenostomy
Watch how an Ohio State researcher is reprogramming skin to take over when other organs fail

Accepting the challenge: Ohio State physician acts to eliminate systemic inequity in health care

Ohio State Expands Its Multidisciplinary Bariatric Surgery Program

Georgios Papachristou, MD, PhD, Brings Pioneering Endoscopic Procedures and Robust Research to Ohio State
New Institute Aims to Transform Care for Patients With Liver and Pancreas Disorders

Ohio State Opens First of its Kind Inflammatory Bowel Disease Center
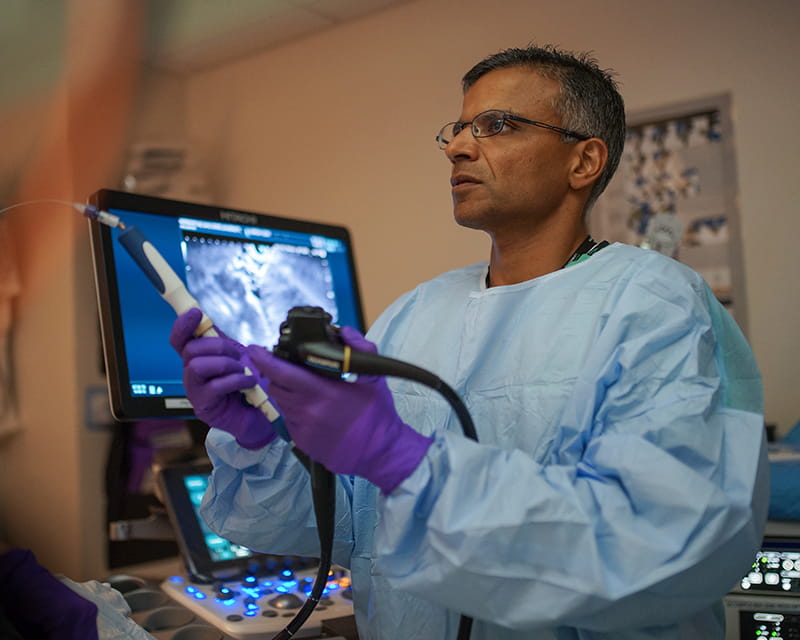
Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy a Critical Tool for Identifying Precancerous and Malignant Pancreatic Cystic Lesions
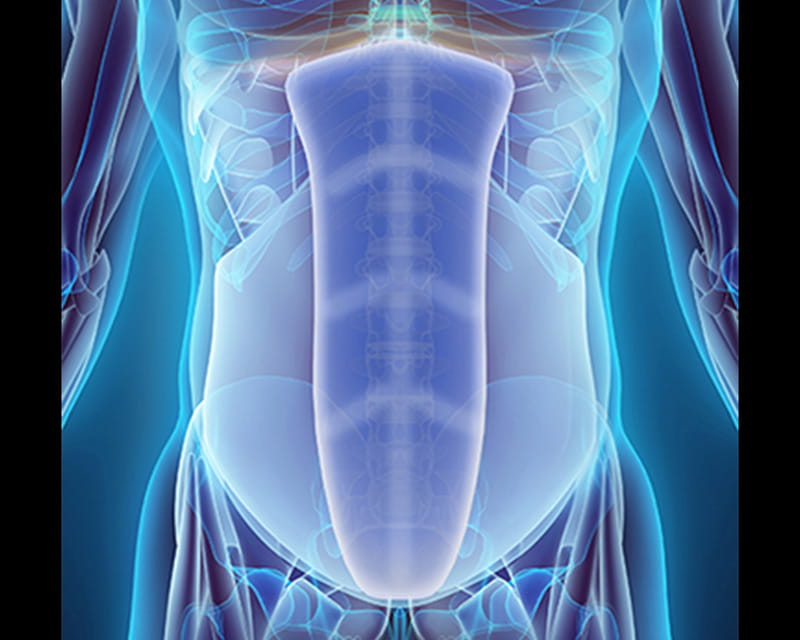
World’s First Center for Abdominal Core Health Rethinks Hernia Treatment

Grant Aims to Identify Disparities in Readmission Rates Among Bariatric Surgery Patients

Colorectal Transitional Care Clinic Coordinates Care for Patients
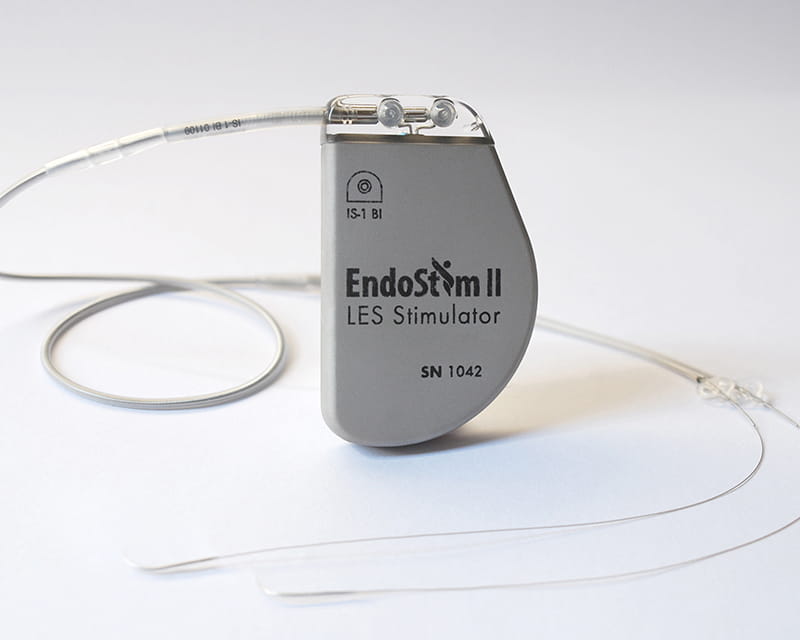
FDA Clinical Trial May Be Promising for Treatment of GERD
Pediatric-to-Adult IBD Transition Program
Study Aims to Identify Ways to DETECT Diabetes Caused by Pancreatic Cancer or Chronic Pancreatitis

Leading the way