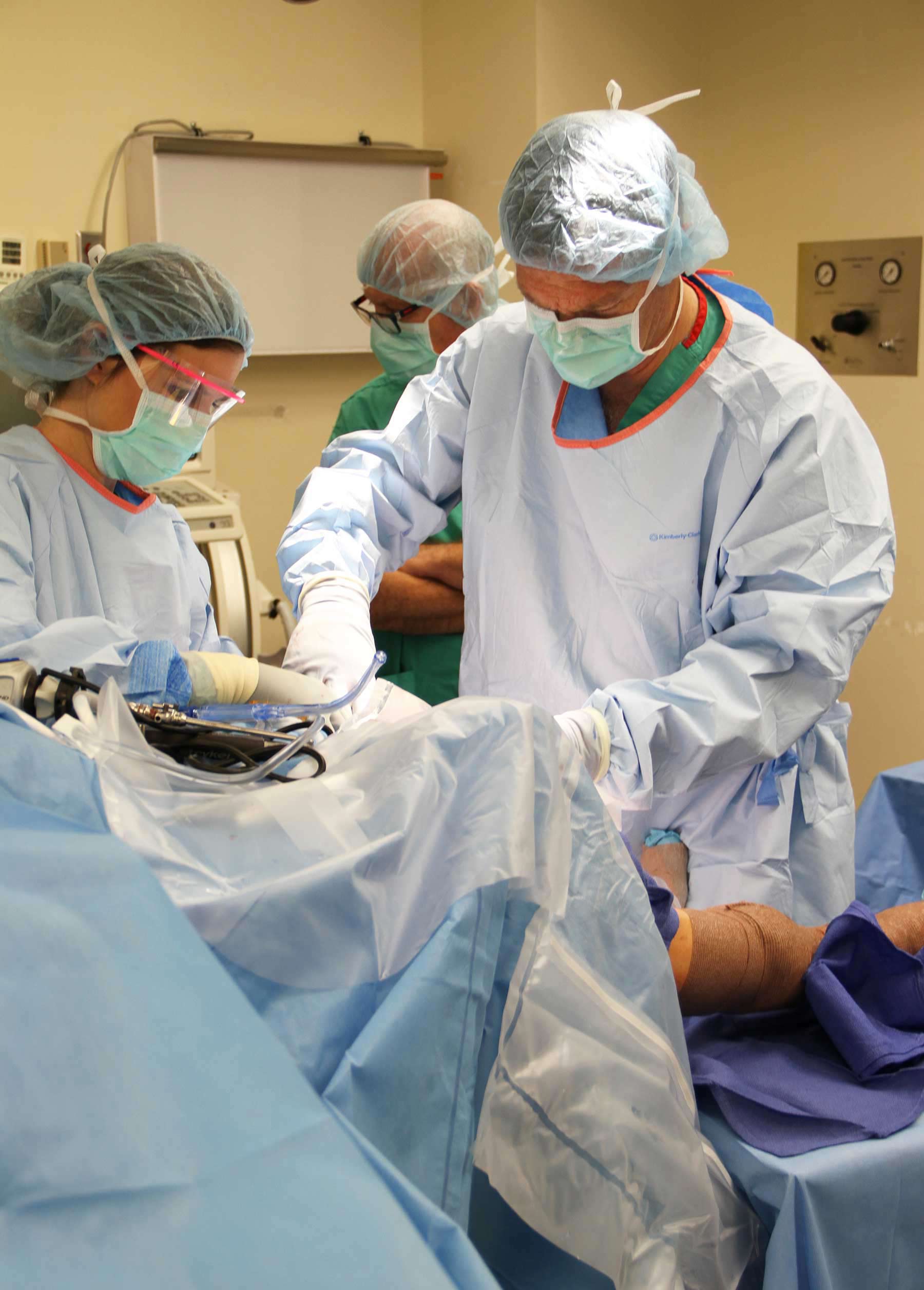
“There haven't been many options for these patients, unfortunately. Once the meniscus is damaged, pain often sets in and can lead to arthritis and the need for knee replacement surgery,” says Christopher Kaeding, MD, (pictured left) the executive director of Ohio State Sports Medicine and the surgeon who implanted the device.“This meniscal implant fills a gap in our treatment for those with meniscus injuries.”
The initial surgery took place during an FDA-approved trial of the NUsurface Meniscus comparing it to the current standard of care for patients with persistent knee pain following meniscus surgery.
The procedure begins with routine arthroscopic preparation of the meniscus, followed by implantation of the plastic meniscus through a small incision. Progressively, the implant will form a customized fit to the patient’s knee contour.
After this procedure, patients no longer need prolonged protected weight bearing or braces. They can wean themselves off crutches when they’re comfortable and progress to normal activities when tolerable.
Currently, an estimated 720,000 patients undergo knee replacement surgery yearly. However, that number is expected to skyrocket to 3.5 million cases by 2030, an increase of nearly 400%.
 “We’re hoping this implant can not only alleviate the pain in these patients, but help them delay or avoid a knee replacement surgery altogether,” Kaeding says.
“We’re hoping this implant can not only alleviate the pain in these patients, but help them delay or avoid a knee replacement surgery altogether,” Kaeding says.
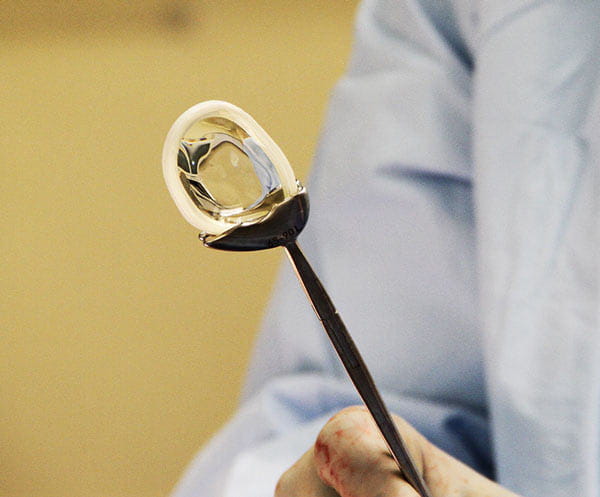
The NUsurface Meniscus Implant (pictured right) is made by Active Implants LLC, the technology leader in cushion-bearing orthopaedics.
