New hope for patients with SMA
The only FDA-approved drug for patients with spinal muscular atrophy is now available
When I first met Emily Roberts 20 years ago, she was just 4 years old. Her parents had noticed she was falling frequently while playing and running around in their home outside of Cleveland. They took her to see a pediatrician, who then referred them to a neurologist, who eventually diagnosed her with spinal muscular atrophy (SMA), a genetic condition that causes nerve cells controlling muscles to break down and die.
Emily and her parents came to see me at The Ohio State University Wexner Medical seeking my assistance as a neurologist and neuromuscular specialist to help manage her disease. They had heard that Ohio State is actively involved in SMA research, and were hopeful for a breakthrough. And now, 20 years later, Emily will be part of a clinical trial at Ohio State studying how well a new drug therapy for SMA slows the progression or even improves the disease in adults.
The drug Spinraza, also known as nusinersen, is the first and only treatment FDA-approved in the United States for SMA.
SMA is a genetic disorder that affects muscle movements and limits a person’s ability to breathe, swallow, walk and use their arms. It’s the leading genetic cause of death in infants, and infants with the most severe form of the disorder rarely live past their second birthday. SMA results from reduced levels of a motor neuron protein, which leads to the selective degeneration of motor neurons in the spinal cord. The disorder affects about 9,000 patients in the United States, and about 1 in 10,000 babies have SMA.
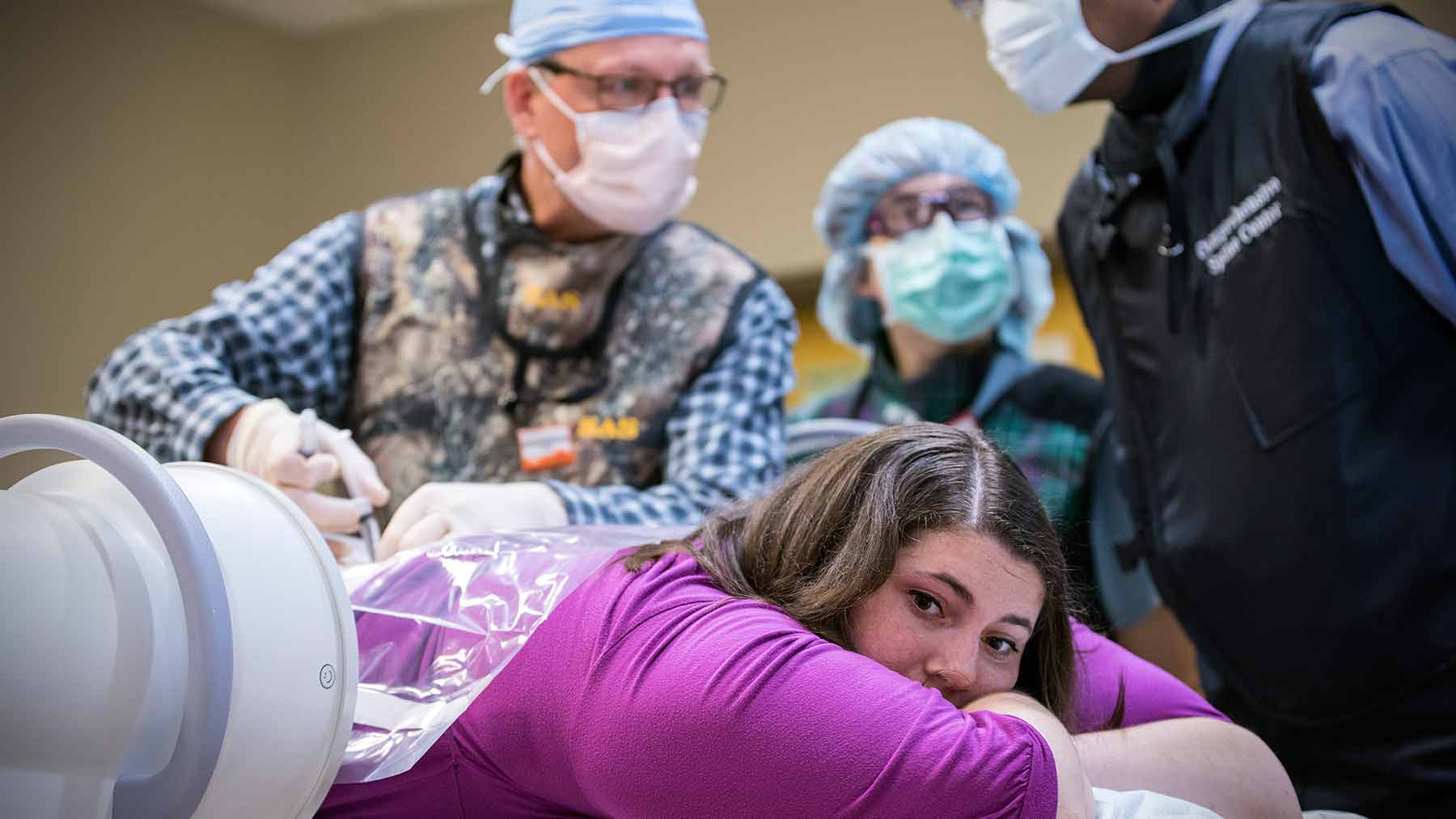
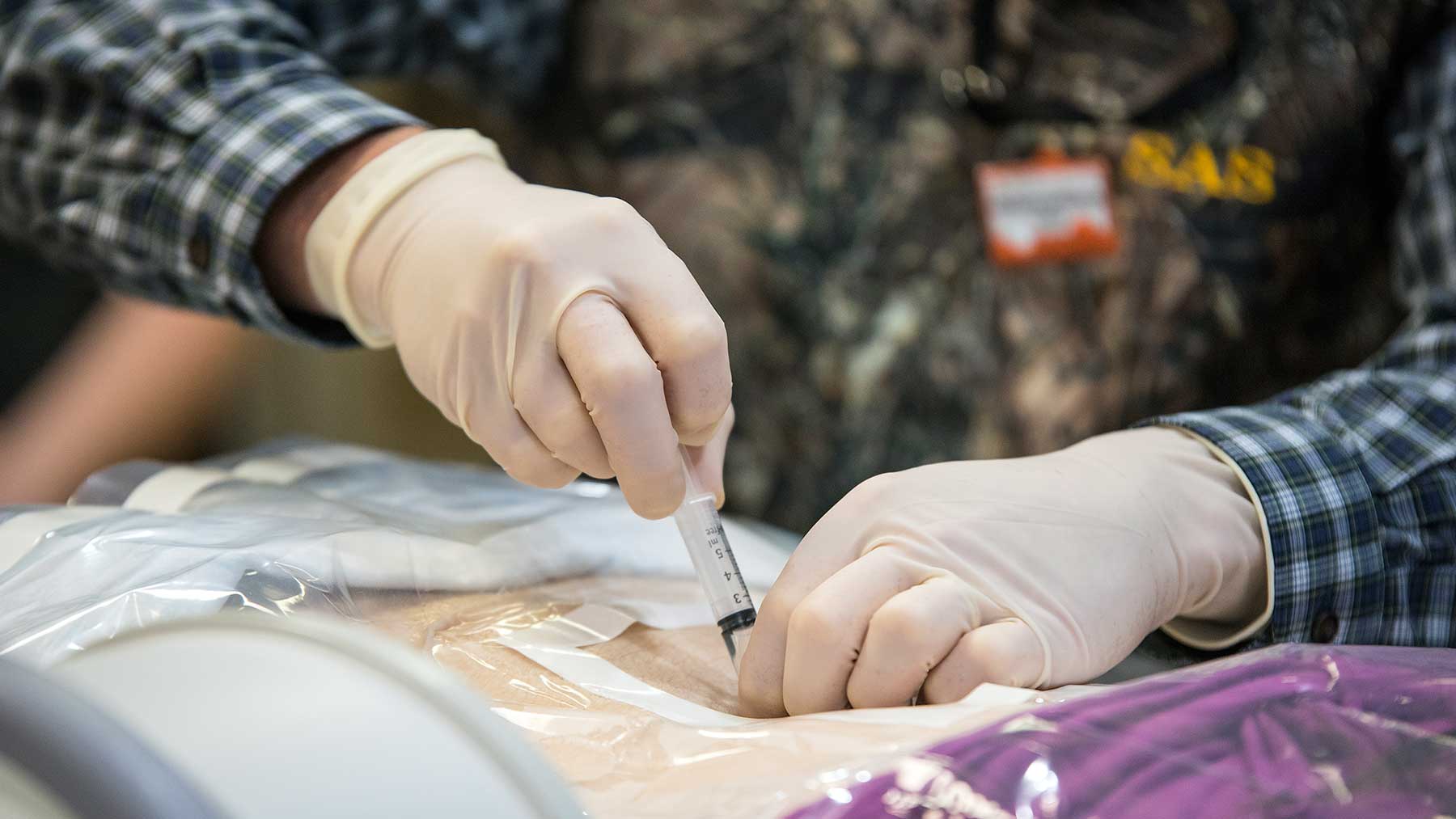
On June 23, Emily, who has a milder form of the disease, became the first patient to receive this new therapy at Ohio State’s Wexner Medical Center. A team of our neurology experts led by Drs. Bakri Elsheikh and Steve Severyn administered the drug via a lumbar injection into her spinal fluid, one of several injections she’ll receive over the next few months. She’ll then return three times a year for additional injections. This treatment is also being offered to infants and young adults at other medical facilities.
 While I’m thrilled to finally have a new therapy to offer my patients after all these years, more work needs to be done to try to cure this disease, so SMA research continues at Ohio State.
While I’m thrilled to finally have a new therapy to offer my patients after all these years, more work needs to be done to try to cure this disease, so SMA research continues at Ohio State.
One of my colleagues, Arthur Burghes, PhD, has successfully proven that reversing a protein deficiency through gene therapy is effective in improving and stabilizing SMA in a large animal model. Some of Burghes’ earlier research to create an SMA mouse model laid the foundation for later clinical trials that resulted in Spinraza’s approval.
Another colleague, Dr. Stephen Kolb, who specializes in researching and treating patients with neuromuscular diseases, led research that is shedding light on the natural history and biomarkers to accelerate clinical trials for infants with SMA.
It remains to be seen how this current research may one day impact Emily, who graduated from The Ohio State University and now lives in Detroit. She’s studying to become a pathologists’ assistant, a job that requires standing for long periods of time during autopsies. She’s hoping this new drug will slow the progression of her disease, so that she’ll still be able to stand and walk, rather than relying more and more on her electric wheelchair as her muscles continue to weaken.
With this new drug, Emily’s future is suddenly much brighter.
John Kissel is chair of the Department of Neurology at The Ohio State University Wexner Medical Center and a professor of neurology at Ohio State's College of Medicine.




